PLGA Particles for Drug Delivery: Advances, Applications, and Future Perspectives
PLGA particles are versatile drug delivery systems composed of biodegradable polymers. With precise control over size, shape, and drug loading capacity, these particles offer tailored release profiles and enhanced therapeutic outcomes. Explore the properties, fabrication techniques, applications, and benefits of PLGA particles in this comprehensive guide.
Further reading

Testimonials
Drug delivery studies
A Dolomite microfluidic system is helping Dr Samar Damiati, Assistant Professor in the Biochemistry Department at King Abdulaziz University in Saudi Arabia, perform drug encapsulation studies.
Application Notes
Continuous Microfluidic Synthesis of PLGA Nanoparticles by Hydrodynamic Flow Focusing
Methodology for fabrication of monodisperse PLGA nanoparticles with sizes ranging from 50 nm to 30 μm using the Hydrodynamic Flow Focusing Method on Dolomite’s API Encapsulation System and a 5 Input Chip.

Application Notes
Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method
Methodology for fabrication of highly monodisperse PLGA beads with sizes ranging from 10 to 45μm using the Droplet Method on Dolomite’s API Encapsulation System and a 3D Flow Focusing Chip.
I. Introduction to PLGA Particles for Drug Delivery
A. Definition and overview of PLGA particles
PLGA particles are biodegradable and biocompatible polymer particles composed of a copolymer of lactic acid and glycolic acid. This unique polymer structure confers several desirable properties, making PLGA an ideal material for drug delivery applications. PLGA particles can be fabricated with precise control over their size, shape, and drug-loading capacity, providing opportunities for tailored drug release profiles and therapeutic outcomes.
B. Importance of drug delivery systems in pharmaceutical research
Effective drug delivery systems are essential in overcoming various challenges associated with conventional drug administration routes. These challenges include poor drug solubility, short half-lives, low bioavailability, and non-specific distribution. By employing drug delivery systems, pharmaceutical researchers aim to enhance therapeutic efficacy, reduce side effects, and improve patient compliance.
C. Applications of PLGA Particles in Drug Delivery
PLGA particles have found extensive applications in a range of drug delivery strategies. One prominent application is the encapsulation and controlled release of drugs within PLGA particles. This allows for sustained drug release over extended periods, ensuring therapeutic levels of the drug are maintained while reducing the frequency of administration. Additionally, PLGA particles enable the targeted delivery of drugs to specific tissues or cells, enhancing treatment precision and minimizing off-target effects.
PLGA particles have been utilized in diverse routes of administration, including oral, injectable, and inhalation delivery systems. They have been employed for the delivery of a wide range of therapeutic agents, including small molecules, proteins, peptides, and nucleic acids. Furthermore, PLGA particles can be functionalized or modified to incorporate targeting ligands, enabling specific interactions with disease sites and enhancing therapeutic efficacy.
D. Benefits of using PLGA particles for drug delivery
The utilization of PLGA particles in drug delivery offers several key benefits. Firstly, PLGA is biodegradable, meaning that over time, the particles break down into lactic acid and glycolic acid, which are metabolized and eliminated from the body. This property ensures the biocompatibility of PLGA particles and reduces the risk of long-term accumulation.
Secondly, the degradation rate of PLGA particles can be modulated by adjusting the polymer composition and molecular weight. This allows for tailored release kinetics, enabling sustained drug release over days to months. Such control is vital in achieving therapeutic drug levels and minimizing the need for frequent dosing.
Thirdly, the versatility of PLGA particles allows for the encapsulation of a wide range of drugs with varying physicochemical properties. This includes hydrophilic and hydrophobic compounds, enabling the delivery of diverse therapeutic agents.
Lastly, PLGA particles can be easily fabricated using various techniques, including emulsion/solvent evaporation, solvent extraction, nanoprecipitation, and microfluidics-based methods. These methods provide control over particle size, shape, and drug loading capacity, facilitating customization according to specific therapeutic requirements.
In summary, PLGA particles offer immense potential as a drug delivery system in pharmaceutical research. Their versatility, biocompatibility, controlled release capabilities, and the ability to encapsulate various drugs make them a promising platform for improving therapeutic outcomes in a range of applications.
II. Properties and Characteristics of PLGA Particles
PLGA particles possess several unique properties and characteristics that make them highly suitable for drug delivery applications. In this section, we will explore the key attributes of PLGA particles, including their biocompatibility, biodegradability, sustained release capabilities, encapsulation efficiency, drug loading capacity, and options for surface modification and functionalization.
A. Biocompatibility and biodegradability of PLGA
One of the fundamental advantages of PLGA particles is their biocompatibility, meaning they are well-tolerated by the body and do not elicit significant adverse reactions. PLGA is a widely accepted and extensively studied polymer that has been approved by regulatory agencies such as the U.S. Food and Drug Administration (FDA) for various biomedical applications.
Moreover, PLGA is biodegradable, meaning it can be broken down into simpler compounds that are metabolized and eliminated from the body. As PLGA particles degrade, they undergo hydrolysis, resulting in the formation of lactic acid and glycolic acid, which are naturally occurring substances. This biodegradability is advantageous, as it eliminates the need for surgical removal of the particles after drug release and reduces the risk of long-term accumulation.
B. Sustain release for controlled drug release
PLGA particles excel in providing controlled and sustained drug release, which is critical for achieving therapeutic efficacy. The degradation of PLGA particles leads to a gradual release of the encapsulated drug over an extended period. The release kinetics can be modulated by adjusting the molecular weight and composition of the PLGA polymer, allowing for precise control of the release rate.
This sustained release feature of PLGA particles offers several benefits. It enables the maintenance of therapeutic drug levels within the desired range over a prolonged duration, reducing the need for frequent dosing. Additionally, it helps to minimize side effects and potential toxicity associated with high drug concentrations.
C. Encapsulation efficiency and drug loading capacity
PLGA particles offer high encapsulation efficiency, which refers to the ability to effectively entrap and retain drugs within the particle matrix. The encapsulation efficiency is influenced by various factors, including the physicochemical properties of the drug, the formulation method, and the characteristics of the PLGA polymer.
Furthermore, PLGA particles exhibit a remarkable drug loading capacity, allowing for the encapsulation of a wide range of therapeutic agents. Both hydrophilic and hydrophobic drugs can be successfully incorporated into PLGA particles, making them versatile carriers for diverse classes of drugs.
D. Surface modification and functionalization options
PLGA particles offer the flexibility of surface modification and functionalization, enabling customization for specific drug delivery applications. The surface of PLGA particles can be modified by attaching targeting ligands, such as antibodies or peptides, to facilitate specific interactions with target cells or tissues. This targeted approach enhances the therapeutic efficacy and reduces off-target effects.
Additionally, surface modification techniques can be employed to improve the stability, circulation time, and cellular uptake of PLGA particles. Surface modifications may include the attachment of polymers, proteins, or other bioactive molecules that can influence the particle’s interactions with biological systems.
In summary, PLGA particles possess desirable properties for drug delivery applications. Their biocompatibility, biodegradability, sustained release capabilities, high encapsulation efficiency, and options for surface modification make them a promising platform for achieving controlled and targeted drug delivery.
III. PLGA Particle Fabrication Techniques
A. Batch method
The batch method is a conventional approach for fabricating PLGA particles and involves several techniques. Let’s explore three commonly used batch methods:
Emulsion/solvent evaporation method
In this technique, PLGA is dissolved in an organic solvent and then mixed with an aqueous phase containing the drug. The vigorous stirring or sonication results in the formation of an emulsion, where the organic phase forms droplets dispersed in the aqueous phase. Subsequently, solvent evaporation leads to the solidification of the polymer and the formation of PLGA particles encapsulating the drug.
Solvent extraction method
This method involves dissolving PLGA and the drug in an organic solvent to form a solution. The solution is then added dropwise to a nonsolvent, such as water or a nonsolvent liquid, resulting in rapid polymer precipitation and the formation of PLGA particles. The particles are subsequently collected and processed to remove any residual solvents.
Nanoprecipitation technique
In this technique, PLGA and the drug are dissolved in an organic solvent and then rapidly injected into an aqueous phase containing a stabilizer. The rapid mixing of the two phases leads to the formation of nanoparticles due to the solvent diffusion and polymer precipitation. The nanoparticles are further processed to remove the solvent and stabilize the particle size.
B. Microfluidics-based techniques for PLGA particle fabrication
Microfluidics is an emerging and highly promising technique for fabricating PLGA particles with precise control over their size, morphology, and drug loading. Microfluidics involves the manipulation of fluids in microscale channels, allowing for precise control of fluid dynamics and the generation of monodisperse particles.
In microfluidic-based techniques, PLGA and the drug solution are separately loaded into different channels of the microfluidic device. The two streams then meet at a junction, where they mix under controlled flow conditions. The rapid mixing in the confined microscale environment promotes the formation of uniformly sized PLGA particles with high monodisproduction
 |
 |
| Single channel droplet production | Hydrodynamic flow focusing |
The advantages of microfluidics-based techniques for PLGA particle fabrication are noteworthy. Firstly, the precisely controlled fluid dynamics in microfluidic devices allow to produce highly monodisperse particles, which is advantageous for consistent drug encapsulation and release profiles.

Secondly, microfluidics enables the formation of particles with precise control over size and morphology. The size of the particles can be easily adjusted by modifying the flow rates or geometry of the microfluidic channels, providing a high level of reproducibility.
 |
 |
| Dolomite PLGA 14µm |
Dolomite PLGA Monodisperse 3um beads |
Thirdly, microfluidic systems offer the potential for continuous and scalable production of PLGA particles. By controlling the flow rates, it is possible to achieve a continuous stream of particles, eliminating the need for batch-by-batch fabrication.


C. Batch method and Microfluidic comparison
While the batch method has been widely utilized for PLGA particle fabrication, microfluidics-based techniques offer several advantages over traditional batch methods. These include enhanced control over particle size, improved monodispersity, precise manipulation of fluid dynamics, and the potential for continuous and scalable production.

| Traditional methods (Batch) | Microfluidic methods | |
|---|---|---|
| API Encapsulation efficiency | up to 30% | up to 99 % |
| Coefficient of variation (CV) | up to 20% | up to 1% |
| Particle size distribution | Broad | Narrow |
| Particle monodispersity | Poor | High |
| Waste | up to 50% | near 0% |
| Reproducibility | Low | High |
| Particle size control | Poor | Precise |
Microfluidics enables the fabrication of monodisperse particles with controlled size distribution, offering better reproducibility and consistent drug release profiles. The ability to precisely control fluid dynamics in microfluidic devices allows for a higher degree of customization in particle design, which is particularly beneficial for targeted drug delivery applications.
Furthermore, the scalability of microfluidic systems provides opportunities for large-scale production, potentially reducing manufacturing costs and improving efficiency compared to batch methods.
In conclusion, while batch methods have been conventionally employed for PLGA particle fabrication, microfluidic-based techniques offer significant advantages in terms of particle size control, monodispersity, fluid dynamics manipulation, and scalability. These advantages make microfluidics a promising method for the precise and reproducible fabrication of PLGA particles for drug delivery applications.
IV. Formulation Considerations and Challenges
A. Selection of appropriate PLGA polymer and its properties
The selection of the appropriate PLGA polymer is crucial for formulating PLGA particles for drug delivery. PLGA polymers with different molecular weights and compositions offer varying properties that can impact the release kinetics, degradation rate, and stability of the particles. Factors to consider include the desired release profile, drug characteristics, and the target tissue or organ. Careful selection of the PLGA polymer ensures compatibility with the drug and desired therapeutic goals.
B. Optimization of formulation parameters
Optimizing the formulation parameters is essential to achieve desired particle characteristics and drug release profiles. Parameters such as polymer concentration, drug-to-polymer ratio, solvent choice, and emulsification techniques can significantly influence the particle size, drug loading, and release kinetics. Through systematic experimentation and characterization, formulation parameters can be fine-tuned to achieve the desired particle properties and drug release behavior.
C. Stability and storage considerations
Stability and storage considerations are critical for maintaining the integrity and performance of PLGA particles. PLGA particles are susceptible to degradation over time, which can affect their drug release kinetics and efficacy. Factors such as temperature, humidity, and exposure to light can accelerate degradation. Proper storage conditions, including refrigeration or freeze-drying, should be implemented to preserve the stability and extend the shelf life of PLGA particles.
D. Overcoming challenges in scale-up and manufacturing, including microfluidics-based approaches
Scaling up the manufacturing process of PLGA particles can present challenges, including maintaining the consistency of particle properties and achieving reproducibility. The transition from laboratory-scale to large-scale production requires careful consideration of process parameters, equipment, and quality control measures. Microfluidics-based approaches offer advantages in scale-up due to their potential for continuous production and precise control over particle size and monodispersity. However, challenges such as device scalability and optimization of flow rates need to be addressed to ensure successful implementation on a larger scale.

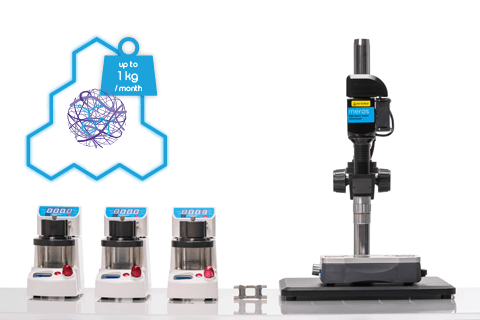
Need to scale-up production?
The highly flexible Telos® system removes the low-throughput limitation of single channel microfluidic chips, enabling liters of droplets, emulsions or particles to be produced per day.
By addressing formulation considerations and challenges, researchers and scientists can optimize the formulation parameters, ensure stability during storage, and overcome hurdles in scale-up and manufacturing. These efforts contribute to the successful development and translation of PLGA-based drug delivery systems for clinical applications.
V. Release Mechanisms and Kinetics of PLGA Particles
PLGA particles exhibit unique release mechanisms and kinetics, making them suitable for controlled and sustained drug delivery. Understanding these mechanisms and the factors influencing drug release is essential for designing effective drug delivery systems. Let’s explore the release mechanisms, kinetics, and the key factors affecting drug release from PLGA particles.
A. Diffusion-controlled drug release
Diffusion-controlled drug release is one of the primary mechanisms observed in PLGA particles. In this mechanism, the drug molecules diffuse through the polymer matrix and are released into the surrounding medium. The rate of drug release is influenced by factors such as the drug’s molecular weight, solubility, and the porosity and tortuosity of the polymer matrix. Smaller drug molecules and more porous polymer matrices generally lead to faster release rates, while larger molecules and denser matrices result in slower release.
B. Degradation-controlled drug release
PLGA particles undergo degradation in physiological conditions, which can contribute to drug release. During degradation, PLGA polymers break down into lactic acid and glycolic acid, which are metabolized and eliminated from the body. The degradation of the polymer matrix creates voids and channels within the particles, allowing for drug diffusion and release. The degradation rate of PLGA can be tuned by modifying the polymer composition, molecular weight, and the presence of copolymers.
C. Factors influencing drug release kinetics
Several factors influence the drug release kinetics from PLGA particles:
- Polymer properties: The molecular weight and composition of the PLGA polymer influence the degradation rate and the release kinetics. Higher molecular weight polymers typically degrade more slowly, leading to prolonged drug release.
- Drug characteristics: Factors such as the drug’s solubility, molecular weight, and hydrophobicity affect its release from PLGA particles. Highly hydrophobic drugs tend to have slower release rates due to their limited solubility in the surrounding aqueous environment.
- Particle properties: The particle size, surface area, and morphology of PLGA particles impact the drug release kinetics. Smaller particles typically exhibit faster release due to their higher surface area-to-volume ratio.
- Polymer/drug ratio: The drug-to-polymer ratio in the formulation influences the drug loading capacity and can affect the release kinetics. Higher drug-to-polymer ratios generally result in faster release rates.
- Environmental factors: The pH, temperature, and presence of enzymes or other biomolecules in the surrounding environment can influence the drug release kinetics by affecting the degradation rate of PLGA particles.
Understanding these factors and their interplay allows researchers to design PLGA-based drug delivery systems with tailored release profiles and optimized therapeutic outcomes.
VI. Advances and Future Directions in PLGA Particle Technology
A. Combination therapy and multi-drug encapsulation
PLGA particles offer a versatile platform for combination therapy, where multiple drugs with different therapeutic actions can be encapsulated within a single particle. This approach allows for synergistic effects, reduced dosage frequency, and improved patient compliance. By incorporating multiple drugs into PLGA particles, combination therapy can be tailored to target specific diseases or overcome drug resistance. The precise control over drug loading and release kinetics provided by PLGA particles enables effective combination therapy for various applications, including cancer treatment and infectious diseases.
B. Nanotechnology and nanoscale PLGA particles
Nanotechnology plays a crucial role in advancing PLGA particle technology. By reducing the particle size to the nanoscale, nanoscale PLGA particles offer unique advantages such as increased surface area, improved cellular uptake, and enhanced drug delivery to specific tissues or cells. Nanoscale PLGA particles can bypass biological barriers, improve bioavailability, and enable targeted drug delivery. Furthermore, surface modification techniques can be employed to functionalize the particles, allowing for active targeting, controlled release, and stimuli-responsive behavior.
C. Targeting strategies for enhanced drug delivery
Targeted drug delivery aims to deliver therapeutics directly to the site of action while minimizing off-target effects. PLGA particles can be engineered to achieve targeted drug delivery through various strategies. These include surface modification with ligands or antibodies that specifically recognize and bind to target receptors or biomarkers, enabling selective accumulation and enhanced uptake at the desired site. Targeting strategies can improve therapeutic efficacy, reduce systemic toxicity, and enhance patient outcomes in a wide range of diseases, including cancer, inflammatory disorders, and cardiovascular conditions.
D. Integration of microfluidics systems for improved PLGA particle fabrication
The integration of microfluidic systems with PLGA particle fabrication has emerged as a promising approach to enhance the precision and efficiency of particle production. Microfluidics offers precise control over fluid dynamics, enabling the production of monodisperse particles with narrow size distributions. The continuous flow nature of microfluidic systems allows for scalable and reproducible manufacturing, addressing challenges associated with batch methods. Microfluidics can facilitate the synthesis of PLGA particles with tailored properties, such as size, morphology, and drug loading, while also enabling the incorporation of functional additives or encapsulation of multiple drugs. The integration of microfluidic systems with PLGA particle fabrication holds great potential for advancing personalized medicine and enabling the translation of PLGA-based drug delivery systems into clinical practice.
System solutions
Dolomite’s Drug (API) Encapsulation Systems are the perfect solution for this pharmaceutical application as they utilize microfluidic methods to directly generate monodisperse particles, beads and emulsions, eliminating the need for further processing. They can be used to generate particles in nano and micro size range, resulting in formulations such as water in oil (w/o) or oil in water (o/w) emulsions.
In conclusion, advances in PLGA particle technology have opened exciting opportunities for combination therapy, nanotechnology, targeted drug delivery, and the integration of microfluidics. These advancements contribute to the development of more efficient and effective drug delivery systems with enhanced therapeutic outcomes. Continued research and innovation in PLGA particle technology will undoubtedly shape the future of drug delivery, paving the way for personalized treatments and improved patient care.
VII. Case Studies and Success Stories
Pioneering microfluidics technologies to treat pregnant populations
A research group at the University of Manitoba is using microfluidics technologies from Dolomite Microfluidics to design and evaluate novel nanoparticle drug delivery systems for the safe and effective treatment of diseases during pregnancy. The laboratory has developed an innovative microfluidics model to mimic the structure of the placenta and is using this system to assess the potential of new nanoparticle-based therapies for serious congenital diseases, such as diaphragmatic hernia.

Read more about the case study here
Microfluidics enables reliable siRNA drug delivery for inflammatory diseases and tumor targeting
Researchers in the Department of Pharmacy at the Ludwig Maximilian University of Munich are using chips from Dolomite Microfluidics to reliably and consistently produce monodisperse particles for targeted delivery of small interfering RNA (siRNA) therapeutics. This microfluidic encapsulation technology is ideal for gene silencing applications in cancer immunology and inflammatory diseases, where siRNA can potentially be used to down-regulate genes associated with these pathologies. Prof. Dr Olivia Merkel, Professor of Drug Delivery, explained: “Nanoencapsulation is a highly efficient way to deliver siRNA to targets across the cellular membrane, protecting it from degradation prior to endocytosis. It is important to deliver the therapy in a controlled and reproducible manner; the particle size has a huge impact on in vivo work, affecting the rate of uptake and clearance.”

Read more about the case study here
Developing microfluidic routes to effective nanoparticle drug delivery systems
A Dolomite Microfluidics’ set-up is helping researchers in the University of Manchester’s Division of Pharmacy and Optometry to enhance drug delivery. Dr Annalisa Tirella explained: “My background is bioengineering, where I gained expertise in biomaterials and fabrication techniques. At the beginning of my career, I focused my research on the design and manufacture of biomaterials to be used as in vitro models for tissue engineering and regenerative medicine applications. Now, I am applying this knowledge of micro-scale techniques to the microfluidic encapsulation of therapeutics in nanoparticles, to offer more efficient and effective targeted drug delivery. Currently, my research group is formulating PLGA drug delivery systems for oncology applications, and liposomes for the co-delivery of fat- and water-soluble therapeutics.”

Read more about the case study here
VIII. Conclusion
In conclusion, PLGA particles have emerged as a versatile and effective platform for drug delivery. With their biocompatibility, controlled release capabilities, and the ability to encapsulate multiple drugs, PLGA particles offer numerous advantages in the field of pharmaceutical research. The integration of microfluidic technology has further enhanced the fabrication process, allowing for precise control, scalability, and reproducibility.
We invite you to explore the potential of microfluidic technology for PLGA particle fabrication. Our microfluidics system provides the tools and capabilities to harness the benefits of precise control and scalability, enabling you to optimize your research and manufacturing processes. By utilizing microfluidics, you can unlock new possibilities in PLGA particle design and enhance the effectiveness of your drug delivery systems.
Take advantage of the opportunities offered by PLGA particles and microfluidics to revolutionize drug delivery. Together, we can shape the future of pharmaceutical research and pave the way for personalized medicine.
References
Cleek, Robert L, et al. “Microparticles of Poly(Dl-Lactic-Co-Glycolic Acid)/Poly(Ethylene Glycol) Blends for Controlled Drug Delivery.” Journal of Controlled Release, vol. 48, no. 2-3, Oct. 1997, pp. 259–268, https://doi.org/10.1016/s0168-3659(97)00052-7. Accessed 19 Oct. 2021.
Han, Felicity Y., et al. “Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading.” Frontiers in Pharmacology, vol. 7, 28 June 2016, www.ncbi.nlm.nih.gov/pmc/articles/PMC4923250/, https://doi.org/10.3389/fphar.2016.00185.
Don’t know where to start?
Get a quick quote!
Leave your contact information and we will be in touch within 24h!
